- Home
- Science
- Our Work
- Air Pollution
- Agriculture, Farming and Pesticides
- Asthma and other Lung Diseases
- Coronavirus Pandemic (COVID-19)
- Exposure to Chemicals and Dust
- Exposure to Nanomaterials
- Human Exposure
- Neurodegenerative Diseases
- Musculoskeletal Disorders
- Occupational Cancer
- Sustainable Working
- Sustainability and Climate Change
- Stress, Wellbeing and Psychosocial Issues
- COVID-19 IOM Study of Face Coverings in Retail Environments
- Styrene Study
- PROTECT COVID-19 National Core Study
- Firefighters and Cancer – IOM Report
- MORtality Study of Former Professional Footballers in England and Wales (MORSE) Study
- Our Scientists
- Our Expertise
- Nano Material Services
- Development and Management of Data and Information Systems and Services
- Ergonomics Design and Evaluation
- Epidemiological Studies & Methods
- Exposure Assessment
- Health Impact Assessment (HIA) and Risk Assessment
- Policy Evaluations
- Study Design and Statistical Analysis
- Systematic Reviews and Meta-analyses
- Toxicology
- Workplace Cluster of Disease
- IOMLIFET
- IOM Scientists Advocate Tighter Standards for Airborne Dust at Work
- Research Project on Work Related Musculoskeletal Disorders
- Styrene Study
- Firefighters and Cancer – IOM Report
- IOM Library
- Contact our Research Experts
- Our Work
- Occupational Hygiene
- Case Studies
- Air Quality Sensors
- COSHH Assessment
- Dust Exposure
- Environmental Management
- Face Fit Testing
- Hand-Arm Vibration
- Indoor Air Monitoring
- Laboratory Animal Allergens
- Legionella Risk Assessment
- Local Exhaust Ventilation
- Noise Monitoring
- Thermal Exposure Monitoring
- Workplace Exposure Limits (WELs)
- Welding Fumes
- Remote Monitoring Services
- Formaldehyde Exposure Monitoring
- Biological Agent Exposure Monitoring in Waste Management
- Chromium VI
- Occupational Hygiene – Quick Quote
- Lab Services
- Asbestos and other Fibres
- Asbestos Sample Testing
- Asbestos Proficiency Testing
- Dust and Crystalline Silica
- Lead in Paint
- Metals, acid anions, acid gases
- Microbiology
- Pharmaceuticals
- Solvents & Other Organic Chemicals
- Hazard Assessment and Toxicology
- Dustiness Testing of Bulk Powders
- Testing the effectiveness of protective coverall and PPE
- Lab Services Quick Quote
- Hospital Ventilation
- Authorising Engineer
- Dentistry Post Lockdown
- Design Review
- Independent Review
- Diathermic pen and Electro surgical tool testing
- Microbiological Monitoring
- Systems Refurbishment and Upgrade
- Validation and Verification Testing
- HSE COVID-19 Spot Check Inspections
- Training
- Contact Our Hospital Ventilation Experts
- Consultancy
- Our Company
- Contact Us
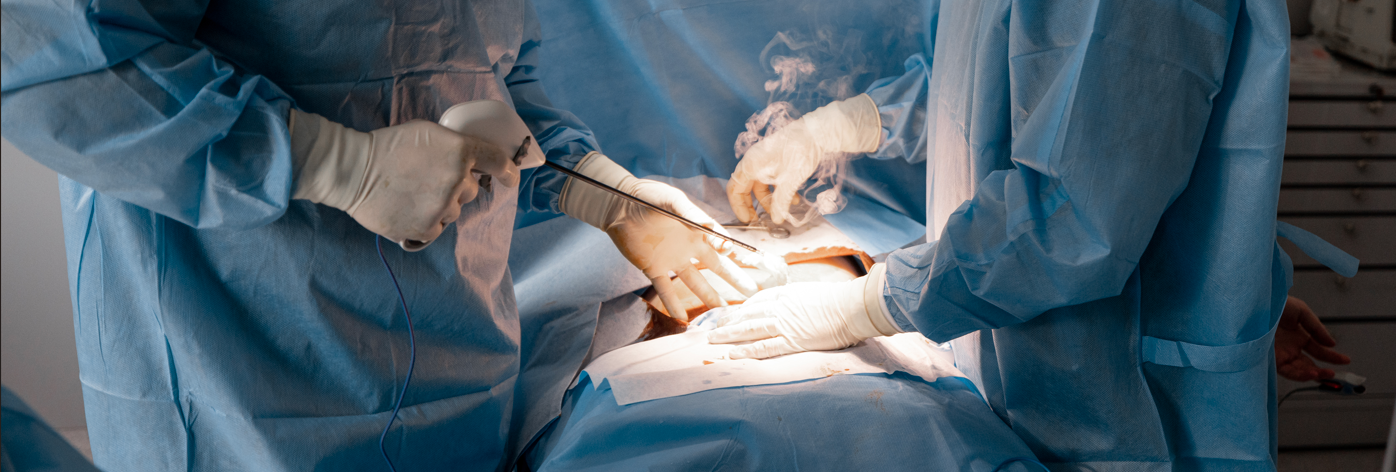
Diathermic Pen and Electro Surgical Tool Testing
Electro surgical tools, including diathermy and laparoscopic instruments, generate surgical plume (surgical smoke) that poses significant health risks. These risks include potential carcinogenic effects, respiratory harm and even viral transmission. To mitigate these dangers, it is crucial to have a plume extraction system (PES) in place, which must be tested every 14 months to comply with COSHH regulations.
Health Risks Posed by Electro Surgical Instruments
Many healthcare professionals are unaware that electro surgical tools are regulated by COSHH. These tools generate surgical plume, which can have serious health implications for those working in healthcare environments. The most concerning risks include:
- Carcinogenic Potential: Some components of the surgical plume are considered to have carcinogenic potential, posing long-term health risks to healthcare workers.
- Acute Health Conditions: Exposure to surgical plume can lead to acute health conditions such as asthma and asthma-like symptoms.
- Respiratory Harm: Diathermy fume can contain numerous toxic chemicals that can cause respiratory harm.
- Viral Transmission: There are reports of possible cases of transmission of human papillomavirus (HPV) from patient to surgeon, highlighting the importance of effective plume extraction.
Where These Risks Are Present
The risks associated with surgical plume are present in all types of PES, whether portable, mobile, stationary, or integrated into other equipment. These systems are used in a variety of settings, including surgical facilities, medical facilities, cosmetic treatment facilities, dental clinics, and veterinary facilities. The ISO standard is broad and states that plume needs to be monitored, as it can be generated by various procedures that rely on the ablation, cauterisation, mechanical manipulation, or thermal desiccation of target tissue.
Compliance with COSHH Regulations
To remain compliant with COSHH regulations, healthcare organizations must ensure that any plume generated by electro surgical tools is properly extracted. This means that the tool must have either an integral extraction system or an external PES located as close to the plume as possible. The control measures must be tested annually, and staff must be trained on using them effectively. Including these LEV systems in risk registers, planned preventative maintenance (PPM), and LEV testing schedules is essential for long-term compliance and staff protection.
How We Help
Comprehensive Testing Services: IOM is one of the few UK companies equipped to test PES systems associated with electro surgical tools. Our thorough examination includes analysing the capture inlet size, shape, location, orientation, and verifying the flow rate.
Risk Assessment and Compliance: We assist in identifying the instruments and equipment that need testing and ensure they are included in your risk register. Our services help you remain compliant with COSHH regulations and protect your staff from health risks.
Trusted Expertise: We are trusted by NHS estates and facilities managers across the country to keep their theatres, procedure, and treatment rooms safe and compliant. Our expertise ensures that your PES systems are effectively maintained and tested.

